|
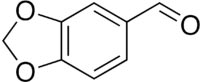
Piperonal
CAS number 120-57-0
General
Synonyms: 3,4-(methylenedioxy)benzaldehyde,
1,3-benzodioxole-5-carboxaldehyde, heliotropin, heliotropine,
piperonaldehyde, protocatechuic aldehyde, piperonyl aldehyde
Molecular Weight: 150.131440 [g/mol]
H-Bond Donor: 0 H-Bond Acceptor: 3
Molecular formula: C8H6O3
CAS No: 120-57-0
EINECS No: 204-409-7
Physical data
Appearance: white crystalline solid
Melting point: 35 - 37 C
Boiling point: 264 C
Vapour density:
Vapour pressure:
Density (g cm-3):
Flash point: 113 C (closed cup)
Explosion limits:
Autoignition temperature:
Water solubility:
Stability
Stable, but air and light sensitive. Combustible. Incompatible with
strong oxidizing agents, bases.
Toxicology
Skin irritant.
ORL-RAT LD50 2700 mg kg IPR-MUS LD50 480 mg kg Risk phrases R38 R52 R53.
Environmental information
Harmful to aquatic organisms - may cause long-term environmental damage.
Personal protection
Safety glasses.
Piperonal
is an aromatic aldehyde that comes as transparent crystals, C8H6O3, and
has a floral odor. It is used as flavoring and in perfume. It can be
obtained by oxidation of piperonyl alcohol or piperic acid. It is also a
minor natural component of the extract of vanilla.
Piperonal's aroma is described as being similar to that of vanillin. It
finds use as a flavoring and in perfume. Piperonal has powerful
aromatherapeutic qualities which appear to elevate mood and general
well-being, and has shown in tests done by the Memorial Sloan-Kettering
Cancer
It may be used in the synthesis of 3,4-methylenedioxyamphetamine (MDA).
In one synthetic path, nitroethane in a glacial acetic acid solution
with an ammonium acetate catalyst yields a substituted nitrostyrene via
a condensation reaction.
Description
Piperonal is found in oils of Spirea ulmaria L., Doriphora sassafras
Endl., and other oils. It is prepared by oxidation of isosafrole. It is
a white crystalline substance with a sweet floral odour resembling
heliotrope and free from safrole by-odour.
PIPERONAL (heliotropine, protocatechuic aldehyde methylene ether),
C8H6O3
an aromatic aldehyde . It is prepared by oxidizing piperic See also:
 ACID
(from the Lat. root ac-, sharp; acere, to be sour) acid with See also:
POTASSIUM [symbol K (from kalium), atomic weight 39.114 0=16)] potassium
permanganate (R . See also: * FITTIG, RUDOLF (1835 ) Fittig, See also:
ANN Ann., 1869, 152, p . 35); by condensing methylene iodide with
protocatechuic aldehyde (R . See also: * WEGSCHEIDER, JULIUS AUGUST
LUDWIG (1771-1849) Wegscheider, Monats., 1893, 14, p . 388) ; or by
oxidizing isosafrol with chromic acid . It forms See also: LONG, GEORGE
(1800-1879) LONG, JOHN DAVIS (1838 ) long colourless crystals which
melt at 370 C. and See also: BOIL boil at 263° C . It has an agreeable
See also: SMELL (connected etymologically with " smoulder " and " smoke
") smell, resembling that of See also: ACID
(from the Lat. root ac-, sharp; acere, to be sour) acid with See also:
POTASSIUM [symbol K (from kalium), atomic weight 39.114 0=16)] potassium
permanganate (R . See also: * FITTIG, RUDOLF (1835 ) Fittig, See also:
ANN Ann., 1869, 152, p . 35); by condensing methylene iodide with
protocatechuic aldehyde (R . See also: * WEGSCHEIDER, JULIUS AUGUST
LUDWIG (1771-1849) Wegscheider, Monats., 1893, 14, p . 388) ; or by
oxidizing isosafrol with chromic acid . It forms See also: LONG, GEORGE
(1800-1879) LONG, JOHN DAVIS (1838 ) long colourless crystals which
melt at 370 C. and See also: BOIL boil at 263° C . It has an agreeable
See also: SMELL (connected etymologically with " smoulder " and " smoke
") smell, resembling that of See also:
HELIOTROPE HELIOTROPE, or TURNSOLE heliotrope, and is much used in See
also: PERFUMERY (Lat. per, through, and fumare, to smoke) perfumery .
It is only slightly soluble in See also: COLD (in O. Eng. cald and
ceald, a word coming ultimately from a root cognate with the Lat. gelu,
gelidus, and common in the Teutonic languages, which usually have two
distinct forms for the substantive and the adjective, cf. Ger. Kolte,
kalt, Dutch koude cold See also: WATER water, but is readily soluble in
See also: ALCOHOL alcohol and in See also: ETHER, (C2H5)2O ether . When
heated with dilute hydrochloric acid to Zoo° C. it yields protocatechuic
aldehyde, C7H6O3, and See also: CARBON (symbol C, atomic weight 12)
carbon . It readily combines with See also: SODIUM [symbol Na, from
Lat. natrium; atomic weight 23.00 (0=16)] sodium bisulphite and with
various bases .
| |
|
Note /Government
Notification: These chemicals are designated as those that are used
in the manufacture of the controlled substances and are important to
the manufacture of the substances. For any (Control Substance)
products Import and Export *** subjected to your country government
laws /control substance ACT.
Information: The information on this web page is provided to help
you to work safely, but it is intended to be an overview of hazards,
not a replacement for a full Material Safety Data Sheet (MSDS). MSDS
forms can be downloaded from the web sites of many chemical
suppliers. ,also that the information on the PTCL Safety web site,
where this page was hosted, has been copied onto many other sites,
often without permission. If you have any doubts about the veracity
of the information that you are viewing, or have any queries, please
check the URL that your web browser displays for this page. If the
URL begins "www.tajapi.com/www/Denatonium Benzoate.htm/" the page is
maintained by the Safety Officer in Physical Chemistry at Oxford
University. If not, this page is a copy made by some other person
and we have no responsibility for it.
The Controlled Substances Act (CSA) was enacted into law by the
Congress of the United States as Title II of the Comprehensive Drug
Abuse Prevention and Control Act of 1970.[1] The CSA is the federal
U.S. drug policy under which the manufacture, importation,
possession, use and distribution of certain substances is regulated.
The Act also served as the national implementing legislation for the
Single Convention on Narcotic Drugs |
|
|
|
|




 ACID
(from the Lat. root ac-, sharp; acere, to be sour) acid with See also:
POTASSIUM [symbol K (from kalium), atomic weight 39.114 0=16)] potassium
permanganate (R . See also: * FITTIG, RUDOLF (1835 ) Fittig, See also:
ANN Ann., 1869, 152, p . 35); by condensing methylene iodide with
protocatechuic aldehyde (R . See also: * WEGSCHEIDER, JULIUS AUGUST
LUDWIG (1771-1849) Wegscheider, Monats., 1893, 14, p . 388) ; or by
oxidizing isosafrol with chromic acid . It forms See also: LONG, GEORGE
(1800-1879) LONG, JOHN DAVIS (1838 ) long colourless crystals which
melt at 370 C. and See also: BOIL boil at 263° C . It has an agreeable
See also: SMELL (connected etymologically with " smoulder " and " smoke
") smell, resembling that of See also:
ACID
(from the Lat. root ac-, sharp; acere, to be sour) acid with See also:
POTASSIUM [symbol K (from kalium), atomic weight 39.114 0=16)] potassium
permanganate (R . See also: * FITTIG, RUDOLF (1835 ) Fittig, See also:
ANN Ann., 1869, 152, p . 35); by condensing methylene iodide with
protocatechuic aldehyde (R . See also: * WEGSCHEIDER, JULIUS AUGUST
LUDWIG (1771-1849) Wegscheider, Monats., 1893, 14, p . 388) ; or by
oxidizing isosafrol with chromic acid . It forms See also: LONG, GEORGE
(1800-1879) LONG, JOHN DAVIS (1838 ) long colourless crystals which
melt at 370 C. and See also: BOIL boil at 263° C . It has an agreeable
See also: SMELL (connected etymologically with " smoulder " and " smoke
") smell, resembling that of See also:


