
Domperidone Maleate
CAS number 99497-03-7
Structure Formula :
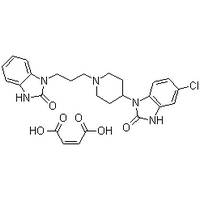
Identification
Synonyms
5-Chloro-1-[1-[3-(2-oxo-1,3-dihydrobenzoimidazol-1-yl)propyl]-4-piperidyl]-1,3-dihydrobenzoimidazol-2-one
maleate
Molecular Structure Domperidone maleate,
5-Chloro-1-[1-[3-(2-oxo-1,3-dihydrobenzoimidazol-1-yl)propyl]-4-piperidyl]-1,3-dihydrobenzoimidazol-2-one
maleate,
CAS Registry Number 99497-03-7
Molecular Formula C22H24ClN5O2.C4H4O4
Molecular Weight 541.99
Appearance: White or almost white powder
Use: Digestive system drugs
Standard: BP/USP/CP
Package: 25KG/Drum
Description
Domperidone is a peripheral dopamine antagonist structurally related to
the butyrophenones with antiemetic and gastroprokinetic properties.
Domperidone effectively increases esophageal peristalsis and lower
esophageal sphincter pressure (LESP), increases gastric motility and
peristalsis, enhances gastroduodenal coordination and consequently
facilitates gastric emptying and decreases small bowel transit time.
The mechanism of action of domperidone is related to its peripheral
dopamine receptor blocking properties. Emesis induced by apomorphine,
hydergine, morphine or levodopa through stimulation of the chemoreceptor
trigger zone (situated outside the blood-brain barrier) can be blocked
by domperidone. There is indirect evidence that emesis is also inhibited
at the gastric level, since domperidone also inhibits emesis induced by
oral levodopa, and local gastric wall concentrations following oral
domperidone are much greater than those of the plasma and other organs.
Domperidone does not readily cross the blood-brain barrier and therefore
is not expected to have central effects.
Domperidone elevates serum prolactin levels but has no effect on
circulating aldosterone levels.
Warnings
In Clinical States: Dopamine receptor blocking agents elevate prolactin
levels; the elevation persists during chronic administration. Tissue
culture experiments indicate that approximately one-third of human
breast cancers are prolactin dependent in vitro, a factor of potential
importance if the prescription of these drugs is contemplated in a
patient with previously detected breast cancer. Although disturbances
such as galactorrhea, amenorrhea, gynecomastia, and impotence have been
reported, the clinical significance of elevated serum prolactin levels
is unknown for most patients. An increase in mammary neoplasms has been
found in rodents after chronic administration of dopamine receptor
blocking agents. Neither clinical studies nor epidemiologic studies
conducted to date, however, have shown an association between chronic
administration of these drugs and mammary tumorigenesis. The available
evidence is considered too limited to be conclusive at this time.
Precautions
In the event that the patient develops galactorrhea and/or
gynecomastia, withdrawal of the drug will result in alleviation of these
symptoms.
Drug Interactions
The concomitant administration of anticholinergic drugs may compromise
the beneficial effects of domperidone.
Since domperidone enhances gastric and small intestinal motility, it may
accelerate absorption of drugs from the small bowel while slowing
absorption of drugs taken up from the stomach, particularly those with
sustained-release or enteric-coated formulations.
Care should be exercised when domperidone is administered in combination
with MAO inhibitors.
The concomitant administration of domperidone maleate with antacids or
H2-receptor blockers does not decrease the absorption of domperidone.
Dosage And Administration
Upper Gastrointestinal Motility Disorders: The usual dosage in
adults is 10 mg orally 3 to 4 times a day, 15 to 30 minutes before meals
and at bedtime if required. In severe or resistant cases the dose may be
increased to a maximum of 20 mg 3 to 4 times a day.
Nausea and Vomiting Associated with Dopamine Agonist Antiparkinsonian
Agents: The usual dosage in adults is 20 mg orally 3 to 4 times a day.
Higher doses may be required to achieve symptom control while titration
of the antiparkinsonian medication is occurring.
|
|




