|
Norpseudoephedrine
CAS number
14838-15-4
 Identification Identification
Synonyms Phenylpropanolamine
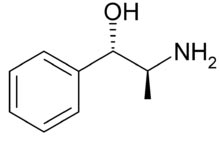
Molecular Structure Norpseudoephedrine,
Phenylpropanolamine,
Molecular Formula C9H13NO
Molecular Weight 151.21
CAS Registry Number 14838-15-4
EINECS 238-900-2
Closely related to ephedrine, cathinone and other amphetamines, it may
contribute to the stimulant effect of Catha edulis, although another
constituent, cathinone appears to show stronger activity.
Norpseudoephedrine (1RS,2RS)-2-Amino-1-phenylpropan-1-ole, C9H13NO, MW
151,21 g/mol. The (1S,2S)-Isomer is called Cathine.
Norpseudoephedrine is a scheduled (S2) substance, used as anorexigenic
(class A.11.3).
As stimulant, Norpseudoephedrine is included in the doping list of the
IOC if a limit value of more than 5 µg/ml in the urine is exceeded.
Norpseudoephedrine is one of the optical isomers of phenylpropanolamine,
an appetite suppressant and decongestant which is possibly associated
with an increased risk of hemorrhagic stroke.
The World Anti-Doping Agency's list of prohibited substances (used for
the Olympic Games among other athletic events) bars cathine in
concentrations of over 5 micrograms per milliliter in urine.
Norpseudoephedrine is a Schedule III drug under the Convention on
Psychotropic Substances.
In order to investigate effects of khat chewing on uteroplacental blood
flow (+) norpseudoephedrine (NPE) infusions were given to 11
anesthetized guinea pigs in late pregnancy (62-66 days) after unilateral
uterine artery ligation at days 30-32.
Regional blood flows were determined with radioactive microspheres.
Mean arterial blood pressure increased with 25% and heart rate with 9%
during NPE infusion. Myoendometrial blood flow was reduced by 31 %.
Placental vascular resistance (PVR) increased by 56% in the control horn
(17 fetuses) and by 82% in the ligated horn (17 fetuses).
This vasoconstriction was counteracted by the systemic vasopressor
response since placental blood flow remained unchanged. When considering
only the 13 growth-retarded fetuses, however, PVR increased by 98% and a
19% reduction of placental blood flow could be demonstrated.
These results suggest that the placenta of the growth-retarded fetus may
be more sensitive to adrenergic stimulation than the normal placenta.
| |
|
Note /Government
Notification: These chemicals are designated as those that are used
in the manufacture of the controlled substances and are important to
the manufacture of the substances. For any (Control Substance)
products Import and Export *** subjected to your country government
laws /control substance ACT.
Information: The information on this web page is provided to help
you to work safely, but it is intended to be an overview of hazards,
not a replacement for a full Material Safety Data Sheet (MSDS). MSDS
forms can be downloaded from the web sites of many chemical
suppliers. ,also that the information on the PTCL Safety web site,
where this page was hosted, has been copied onto many other sites,
often without permission. If you have any doubts about the veracity
of the information that you are viewing, or have any queries, please
check the URL that your web browser displays for this page. If the
URL begins "www.tajapi.com/www/Denatonium Benzoate.htm/" the page is
maintained by the Safety Officer in Physical Chemistry at Oxford
University. If not, this page is a copy made by some other person
and we have no responsibility for it.
The Controlled Substances Act (CSA) was enacted into law by the
Congress of the United States as Title II of the Comprehensive Drug
Abuse Prevention and Control Act of 1970.[1] The CSA is the federal
U.S. drug policy under which the manufacture, importation,
possession, use and distribution of certain substances is regulated.
The Act also served as the national implementing legislation for the
Single Convention on Narcotic Drugs |
|
|
|
|




 Identification
Identification


