
|
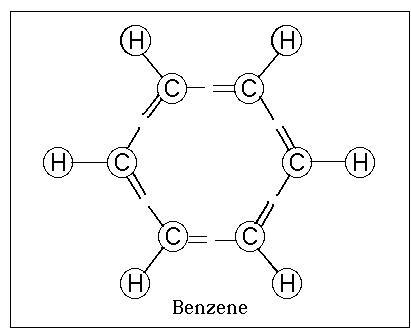
Benzene
|
|
CAS number : 71-43-2
Molecular formula : C6H6
Molar mass : 78.11 g mol−1
Appearance : Colorless liquid
Density : 0.8786 g/cm3
Melting point : 5.5 °C, 279 K, 42 °F
Boiling point : 80.1 °C, 353 K, 176 °F
Solubility in water : 0.8 g/L (25 °C)
Viscosity : 0.652 cP at 20 °C
Dipole moment : 0 D |
Benzene, or benzol, is an organic
chemical compound with the molecular formula C6H6. It is sometimes
abbreviated Ph–H. Benzene is a colorless and highly flammable liquid
with a sweet smell and a relatively high melting point. Because it is a
known carcinogen, its use as an additive in gasoline is now limited, but
it is an important industrial solvent and precursor in the production of
drugs, plastics, synthetic rubber, and dyes. Benzene is a natural
constituent of crude oil, and may be synthesized from other compounds
present in petroleum. Benzene is an aromatic hydrocarbon and the second
[n]-annulene ([6]-annulene), a cyclic hydrocarbon with a continuous pi
bond.
Production
Trace amounts of benzene may result whenever carbon-rich materials
undergo incomplete combustion. It is produced in volcanoes and forest
fires, and is also a component of cigarette smoke. Benzene is a
principal component of combustion products produced by the burning of
PVC (polyvinyl chloride).
Until World War II, most benzene was produced as a by-product of coke
production (or "coke-oven light oil") in the steel industry. However, in
the 1950s, increased demand for benzene, especially from the growing
plastics industry, necessitated the production of benzene from
petroleum. Today, most benzene comes from the petrochemical industry,
with only a small fraction being produced from coal.
Four chemical processes contribute to industrial benzene production:
catalytic reforming, toluene hydrodealkylation, toluene
disproportionation, and steam cracking. In the US, 50% of benzene comes
from catalytic reforming and 25% from steam cracking. In Western Europe,
50% of benzene comes from steam cracking and 25% from catalytic
reforming.
Uses
In the 19th and early-20th centuries, benzene was used as an after-shave
lotion because of its pleasant smell. Prior to the 1920s, benzene was
frequently used as an industrial solvent, especially for degreasing
metal. As its toxicity became obvious, benzene was supplanted by other
solvents, especially toluene (methyl benzene), which has similar
physical properties but is not as carcinogenic.
In 1903, Ludwig Roselius popularized the use of benzene to decaffeinate
coffee. This discovery led to the production of Sanka (the letters "ka"
in the brand name stand for kaffein). This process was later
discontinued. Benzene was historically found as a significant component
in many consumer products such as Liquid Wrench, Testors model cement,
several paint strippers, rubber cements, spot removers and other
hydrocarbon-containing products. Some, like Testors, ceased manufacture
of its benzene formula about 1950 while others continued to use benzene
as a component or significant contaminant until the late 1970s when
leukemia deaths were found associated with Goodyear's Pliofilm
production operations in Ohio. Until the late 1970s, many hardware
stores, paint stores, and other retail outlets sold benzene in small
cans, such as quart size, for general-purpose use. Many students were
exposed to benzene in school and university courses while performing
laboratory experiments with little or no ventilation in many cases. This
very dangerous practice has been almost totally eliminated.
As a gasoline (petrol) additive, benzene increases the octane rating and
reduces knocking. Consequently, gasoline often contained several percent
benzene before the 1950s, when tetraethyl lead replaced it as the most
widely-used antiknock additive. With the global phaseout of leaded
gasoline, benzene has made a comeback as a gasoline additive in some
nations. In the United States, concern over its negative health effects
and the possibility of benzene entering the groundwater have led to
stringent regulation of gasoline's benzene content, with limits
typically around 1%.
European petrol specifications now contain the same 1% limit on benzene
content. The United States Environmental Protection Agency has new
regulations that will lower the benzene content in gasoline to 0.62% in
2011.
Benzene is an aromatic hydrocarbon that is produced by the burning of
natural products. It is a component of products derived from coal and
petroleum and is found in gasoline and other fuels. Benzene is used in
the manufacture of plastics, detergents, pesticides, and other
chemicals. Research has shown benzene to be a carcinogen
(cancer-causing). With exposures from less than five years to more than
30 years, individuals have developed, and died from, leukemia. Long-term
exposure may affect bone marrow and blood production. Short-term
exposure to high levels of benzene can cause drowsiness, dizziness,
unconsciousness, and death.
 Note:
These API/ chemicals are designated as
those that are used in the manufacture of the controlled substances and
are important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your country
government laws /control substance ACT. Note:
These API/ chemicals are designated as
those that are used in the manufacture of the controlled substances and
are important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your country
government laws /control substance ACT.
Note /Government Notification:
N/A
|
|









