
|
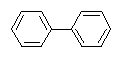
Biphenyl Phenyl benzene
|
|
CAS number : 92-52-4
Molecular formula : C12H10
Molar Weight : 154.21 g mol−1
Density : 0.992 g/cm3
Melting point : 68.93 °C
Boiling point : 256 °C
Solubility in water : Insoluble
Dangerous for the environment : (N)
Flash point : 113 °C
Auto ignition temperature : 540 °C |
Biphenylis an organic compound that
forms colorless crystals. It has a distinctively pleasant smell.
Biphenyl is an aromatic hydrocarbon with a molecular formula (C6H5)2. It
is notable as a starting material for the production of polychlorinated
biphenyls (PCBs), which were once widely used as dielectric fluids and
heat transfer agents. Biphenyl is also an intermediate for the
production of a host of other organic compounds such as emulsifiers,
optical brighteners, crop protection products, and plastics.
Properties
Biphenyl occurs naturally in coal tar, crude oil, and natural gas and
can be produced from these sources by distillation. Biphenyl is
insoluble in water, but soluble in typical organic solvents. The
biphenyl molecule consists of two connected phenyl rings. Lacking
functionalization, it is not very reactive.
Biological aspects
Biphenyl prevents the growth of molds and fungus, and is therefore used
as a preservative particularly in the preservation of citrus fruits
during transportation.
It is mildly toxic, but can be degraded biologically by conversion into
nontoxic compounds. Some bacteria are able to hydroxylate biphenyl and
its polychlorinated biphenyls
Biphenyl compounds
Substituted biphenyls can be prepared synthetically by various coupling
reactions including the Suzuki reaction and the Ullmann reaction and
have many uses. Polychlorinated biphenyls were once used as cooling and
insulating fluids and polybrominated biphenyls are flame retardants. The
biphenyl motif also appears in drugs such as Valsartan and Telmisartan.
Hazards Identification
Inhalation:
Inhalation of dust or vapors can irritate the mucous membranes and
respiratory tract. Other symptoms may parallel those from ingestion
exposure.
Ingestion:
Exerts toxic effects on the central nervous system and liver. Symptoms
may include headache, diffuse gastro-intestinal pain, nausea, numbness,
body aches, and general fatigue.
Skin Contact:
May cause irritation. May be absorbed through the skin with symptoms
paralleling those from ingestion exposure. May cause allergic reaction
in sensitive individuals.
Eye Contact:
Vapors and dust cause eye irritation.
Chronic Exposure:
Chronic exposure may cause peripheral nerve damage and liver injury.
Aggravation of Pre-existing Conditions:
No information found.
First Aid Measures
Inhalation:
Remove to fresh air. If not breathing, give artificial respiration. If
breathing is difficult, give oxygen. Get medical attention.
Ingestion:
Induce vomiting immediately as directed by medical personnel. Never give
anything by mouth to an unconscious person. Get medical attention.
Skin Contact:
Immediately flush skin with plenty of soap and water for at least 15
minutes while removing contaminated clothing and shoes. Get medical
attention. Wash clothing before reuse. Thoroughly clean shoes before
reuse.
Eye Contact:
Immediately flush eyes with plenty of water for at least 15 minutes,
lifting lower and upper eyelids occasionally. Get medical attention
immediately.
Handling and Storage
Keep in a tightly closed container, stored in a cool, dry, ventilated
area. Protect against physical damage. Isolate from incompatible
substances. Containers of this material may be hazardous when empty
since they retain product residues (dust, solids); observe all warnings
and precautions listed for the product.

 Note:
These API/ chemicals are designated as
those that are used in the manufacture of the controlled substances and
are important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your country
government laws /control substance ACT. Note:
These API/ chemicals are designated as
those that are used in the manufacture of the controlled substances and
are important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your country
government laws /control substance ACT.
Note /Government Notification:
N/A
|
|









