
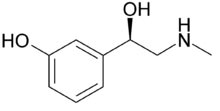
Systematic (IUPAC) name
(R)-3-[-1-hydroxy-2-(methylamino)ethyl]phenol
 Phenylephrine
or Neo-Synephrine is an α1-adrenergic receptor agonist used primarily as
a decongestant, as an agent to dilate the pupil, and to increase
blood pressure. Phenylephrine has recently been marketed as a substitute
for pseudoephedrine but there are recent claims that oral phenylephrine
may be no more effective as a decongestant than a placebo. Phenylephrine
or Neo-Synephrine is an α1-adrenergic receptor agonist used primarily as
a decongestant, as an agent to dilate the pupil, and to increase
blood pressure. Phenylephrine has recently been marketed as a substitute
for pseudoephedrine but there are recent claims that oral phenylephrine
may be no more effective as a decongestant than a placebo.
Chemical data
Formula : C9H13NO2
Mol. mass : 167.205 g/mol
SMILES : eMolecules
 Pharmacokinetic
data Pharmacokinetic
data
Bioavailability 38% through GI tract
Protein binding 95%
Metabolism Hepatic (monoamine oxidase)
Half life 2.1 to 3.4 hours
Routes Oral, intranasal, ophthalmic, intravenous, intramuscular
Phenylephrine is used to relieve nasal discomfort
caused by colds, allergies, and hay fever. It is also used to relieve
sinus congestion and pressure. Phenylephrine will relieve symptoms but
will not treat the cause of the symptoms or speed recovery.
Phenylephrine is in a class of medications called nasal decongestants.
It works by reducing swelling of the blood vessels in the nasal
passages.
Uses
Phenylephrine comes as a tablet, a liquid, or a dissolving strip to take
by mouth. It is usually taken every 4 hours as needed. Follow the
directions on your prescription label or the package label carefully,
and ask your doctor to explain any part you do not understand. Take
phenylephrine exactly as directed.
Phenylephrine comes alone and in combination with other medications.
Check nonprescription cough and cold product labels carefully before
using two or more products at the same time. These products may contain
the same active ingredient(s) and taking them together could cause you
to receive an overdose. This is especially important if you will be
giving cough and cold medications to a child.
If you are giving phenylephrine or a combination product that contains
phenylephrine to a child, read the package label carefully to be sure
that it is the right product for a child of that age. Do not give
phenylephrine products that are made for adults to children.
Before you give a phenylephrine product to a child, check the package
label to find out how much medication the child should receive. Give the
dose that matches the child's age on the chart. Ask the child's doctor
if you don't know how much medication to give the child.
Phenylephrine may cause side effects.
* nervousness
* dizziness
* sleeplessness
Phenylephrine is sometimes used as a vasopressor to increase the
blood pressure in unstable patients with hypotension. Such use is
more common in anesthesia or critical-care practices; phenylephrine is
especially useful in counteracting the hypotensive effect of epidural
and subarachnoid anesthetics. It also has the advantage of not being
inotropic or chronotropic, and so it strictly elevates the blood
pressure without increasing the heart rate or contractility (reflex
bradycardia may result from the blood pressure increase, however). This
is especially useful if the heart is already tachycardic and/or has a
cardiomyopathy. The elimination half life of phenylephrine is about 2.5
to 3 hours.
Substitute for pseudoephedrine
Pseudoephedrine and phenylephrine are both used as decongestants; and,
until recently, pseudoephedrine was much more commonly available in the
United States. This has changed because provisions of the Combat
Methamphetamine Epidemic Act of 2005 placed restrictions on the sale of
pseudoephedrine products in order to prevent the clandestine manufacture
of methamphetamine. Since 2004, phenylephrine has been increasingly
marketed as a substitute for pseudoephedrine; some manufacturers have
changed the active ingredients of products to avoid the restrictions on
sales. Phenylephrine has been off patent for some time, and there are
many generic brands available.
Clinical Pharmacology
Phenylephrine is sympathomimetic vasoconstrictor that has been used as a
nasal decongestant for many years . Phenylephrine has one chiral centre
and can exist as either the S(+) or R(-) enantiomer. The R(-) enantiomer
is the one used in products containing phenylephrine. It is a relatively
selective alpha-adrenoceptor agonist. The majority of the
sympathomimetic action is due to direct stimulation of the adrenoceptors
and relatively little is due to an indirect effect via release of
noradrenaline [1]. Its pressor action is weaker than that of
noradrenaline but of longer
duration [4]. At therapeutic doses, it does not cause significant
stimulation of the central nervous system.
Sympathomimetic decongestants reduce the nasal congestion due to
increased nasal blood flow associated with colds and influenza. This
effect forms the therapeutic basis for their use in these conditions.
Hypertensive patients should be aware of the possible side effects of
nonprescription medications on blood pressure control. For absolute
safety no adrenergic agents should be used. However, when required,
phenylephrine is the safest of these agents. Studies assessing the
hypertensive effect of oral phenylephrine in normotensive volunteers
have demonstrated that the minimal dose required to elicit an increase
in blood pressure is approximately 50 mg that is five times the
therapeutic dose.
Doses in excess of 120 mg are required to elicit a significant effect on
blood pressure. A recent study in normotensive volunteers demonstrated
that following administration of a cold relief product containing
phenylephrine 10 mg and caffeine 60 mg, there was a small but
statistically significant increase in total peripheral resistance but no
consistent effect on other cardiovascular parameters including heart
rate and blood
pressure.
Interactions
The coadministration of Monoamine Oxidase Inhibitors (MAOIs) or
tricyclic antidepressants and an indirect or mixed-acting
sympathomimetic may result in a hypertensive crisis. Direct-acting
sympathomimetics appear to interact minimally, if at all [16 - Drug
Interaction Facts. 4th edition]. Such concomitant use is clearly
identified as a contra-indication on the labelling of all phenylephrine-containing
products and the appropriate warnings are provided. Additionally
sympathomimetics may reduce the efficacy of beta-blocking and
anti-hypertensive drugs. Conditions where these drugs are used are
contra-indicated for the product.
Storage/Stability/Compatibility
The injectable product should be stored protected from light. Do not use
solutions if they are brown or contain a precipitate. Oxidation of the
drug can occur without a color change. To protect against oxidation, the
air in commercially available ampules for injection is replaced with
nitrogen and a sulfite added.
Phenylephrine is reported to be compatible with all commonly used IV
solutions and the following drugs: chloramphenicol sodium succinate,
dobutamine HCl, lidocaine HCl, potassium chloride, and sodium
bicarbonate. While stated to be incompatible with alkalies, it is
stable with sodium bicarbonate solutions. Phenylephrine is reported to
be incompatible with ferric salts, oxidizing agents, and metals.
 Note:
These API/ chemicals are designated as
those that are used in the manufacture of the controlled substances and
are important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your country
government laws /control substance ACT. Note:
These API/ chemicals are designated as
those that are used in the manufacture of the controlled substances and
are important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your country
government laws /control substance ACT.
Note /Government Notification:
These chemicals are designated as those that are used in the manufacture
of the controlled substances and are important to the manufacture of the
substances. For any (Control Substance) products Import and Export ***
subjected to your country government laws /control substance ACT.
Information: The information on this web page is provided to help you
to work safely, but it is intended to be an overview of hazards, not a
replacement for a full Material Safety Data Sheet (MSDS). MSDS forms can
be downloaded from the web sites of many chemical suppliers. ,also that
the information on the PTCL Safety web site, where this page was hosted,
has been copied onto many other sites, often without permission. If you
have any doubts about the veracity of the information that you are
viewing, or have any queries, please check the URL that your web browser
displays for this page. If the URL begins "www.tajapi.com/www/Denatonium
Benzoate.htm/" the page is maintained by the Safety Officer in Physical
Chemistry at Oxford University. If not, this page is a copy made by some
other person and we have no responsibility for it.
The Controlled Substances Act (CSA) was enacted into law by the Congress
of the United States as Title II of the Comprehensive Drug Abuse
Prevention and Control Act of 1970.[1] The CSA is the federal U.S. drug
policy under which the manufacture, importation, possession, use and
distribution of certain substances is regulated. The Act also served as
the national implementing legislation for the Single Convention on
Narcotic Drugs.
|
|




 Phenylephrine
or Neo-Synephrine is an α1-adrenergic receptor agonist used primarily as
a decongestant, as an agent to dilate the pupil, and to increase
blood pressure. Phenylephrine has recently been marketed as a substitute
for pseudoephedrine but there are recent claims that oral phenylephrine
may be no more effective as a decongestant than a placebo.
Phenylephrine
or Neo-Synephrine is an α1-adrenergic receptor agonist used primarily as
a decongestant, as an agent to dilate the pupil, and to increase
blood pressure. Phenylephrine has recently been marketed as a substitute
for pseudoephedrine but there are recent claims that oral phenylephrine
may be no more effective as a decongestant than a placebo. Pharmacokinetic
data
Pharmacokinetic
data




