
Phenyl Propanolamine BP/USP
 Item
Number : PH157 Item
Number : PH157
CAS Number : 154-41-6
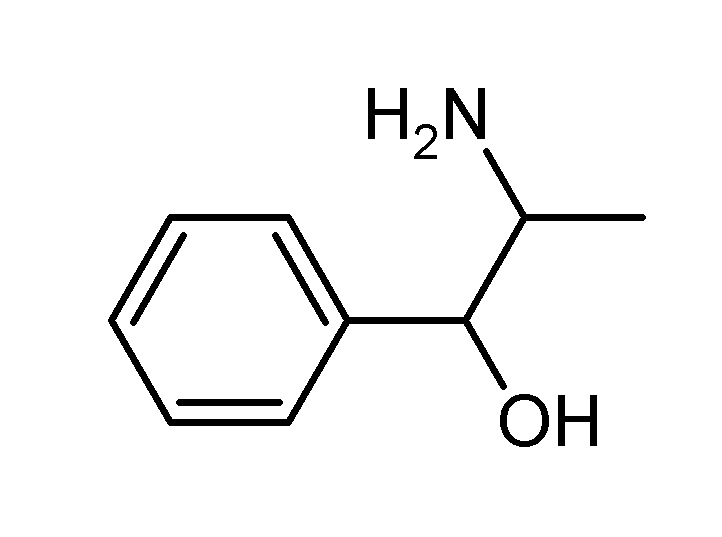
Molecular Formula : C9H13NO.HCl
Molecular Weight : 187.67
Synonyms (1RS,2SR)-2-Amino-1-phenylpropan-1-ol Hydrochloride; (+/-)-Norephedrine
Hydrochloride
Phenylpropanolamine is a decongestant. It works by constricting
(shrinking) blood vessels (veins and arteries) in your body.
Constriction of blood vessels in your sinuses, nose, and chest allows
drainage of those areas, which decreases congestion.
Phenylpropanolamine is used to treat the congestion associated with
allergies, hay fever, sinus irritation, and the common cold.
Phenylpropanolamine also causes a decrease in appetite and is used in
some over-the-counter diet aids.
Phenylpropanolamine has been associated with an increased risk of
hemorrhagic stroke (bleeding into the brain or into tissue surrounding
the brain) in women. Men may also be at risk. Although the risk of
hemorrhagic stroke is low, the U.S. Food and Drug Administration (FDA)
recommends that consumers not use any products that contain
phenylpropanolamine.
Additional Information
DEA scheduled list 1 chemical. Subject to procurement quota
requirements
Phenylpropanolamine
(PPA; Accutrim, Dexatrim), also knwon as
norephedrine and oxyamphetamine, is a
psychoactive drug of the
phenethylamine and
amphetamine
chemical classes which is used as a
stimulant,
decongestant, and
anorectic agent.[1][2]
It is commonly used in
prescription and
over-the-counter
cough and cold preparations. In
veterinary medicine, it is used to control
urinary incontinence in dogs under
trade names Propalin and Proin.
PPA acts as a
potent and
selective
releasing agent of
norepinephrine and
epinephrine, or as a
norepinephrine releasing agent (NRA). It also acts as a
dopamine releasing agent (DRA) to a lesser extent.
INTERACTIONS WITH OTHER DRUGS
In some cases of urinary incontinence, phenylpropanolamine is used in
combination with diethylstilbesterol (an estrogen). No harmful drug
interactions are expected with this combination.
Phenylpropanolamine should not be used with L-Deprenyl (Anipryl) due to
resulting unpredictable fluctuations in blood pressure.
It is recommended that phenylpropanolamine be withdrawn for 2 weeks
preceding the use of L-Deprenyl.
An increased risk of hypertension can also occur if phenylpropanolamine
is given in conjunction with tricyclic antidepressants (such as
amitriptyline), non-steroidal anti-inflammatory drugs (NSAIDs), or
amitraz (active ingredient of the Preventic tick control collar and
canine Promeris, a flea control product).
Storage Information
LIGHT SENSITIVE: Keep tightly closed in light-resistant containers.
The Food and Drug Administration (FDA) has taken
steps to remove phenylpropanolamine from all drug products and has
issued a public health advisory concerning phenylpropanolamine
hydrochloride. This drug is an ingredient used in many over-the-counter
(OTC) and prescription cough and cold medications as a decongestant and
in over-the-counter weight loss products. Phenylpropanolamine has been
found to increase the risk of hemorrhagic stroke (bleeding into the
brain or into tissue surrounding the brain) in women. Men may also be at
risk. Although the risk of hemorrhagic stroke is very low, FDA
recommends that consumers not use any products that contain
phenylpropanolamine.
Summary of Interactions with Vitamins, Herbs, and Foods
In some cases, an herb or supplement may appear in more than one
category, which may seem contradictory. For clarification, read the full
article for details about the summarized interactions.
 Note:
These API/ chemicals are designated as
those that are used in the manufacture of the controlled substances and
are important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your country
government laws /control substance ACT. Note:
These API/ chemicals are designated as
those that are used in the manufacture of the controlled substances and
are important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your country
government laws /control substance ACT.
Note /Government Notification:
These chemicals are designated as those that are used in the manufacture
of the controlled substances and are important to the manufacture of the
substances. For any (Control Substance) products Import and Export ***
subjected to your country government laws /control substance ACT.
Information: The information on this web page is provided to help you
to work safely, but it is intended to be an overview of hazards, not a
replacement for a full Material Safety Data Sheet (MSDS). MSDS forms can
be downloaded from the web sites of many chemical suppliers. ,also that
the information on the PTCL Safety web site, where this page was hosted,
has been copied onto many other sites, often without permission. If you
have any doubts about the veracity of the information that you are
viewing, or have any queries, please check the URL that your web browser
displays for this page. If the URL begins "www.tajapi.com/www/Denatonium
Benzoate.htm/" the page is maintained by the Safety Officer in Physical
Chemistry at Oxford University. If not, this page is a copy made by some
other person and we have no responsibility for it.
The Controlled Substances Act (CSA) was enacted into law by the Congress
of the United States as Title II of the Comprehensive Drug Abuse
Prevention and Control Act of 1970.[1] The CSA is the federal U.S. drug
policy under which the manufacture, importation, possession, use and
distribution of certain substances is regulated. The Act also served as
the national implementing legislation for the Single Convention on
Narcotic Drugs.

|
|




 Item
Number : PH157
Item
Number : PH157




