
Cefotaxime Sodium
CAS Registry Number is 64485-93-4.
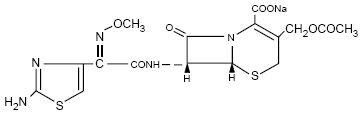
cefotaxime sodiumis a semisynthetic, broad spectrum cephalosporin
antibiotic for parenteral administration. It is the sodium salt of
7-[2-(2-amino-4-thiazolyl)
glyoxylamido]-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo [4.2.0]
oct-2-ene-2-carboxylate 72 (Z)-(o-methyloxime), acetate (ester).
CLAFORAN contains approximately 50.5 mg (2.2 mEq) of sodium per gram of
cefotaxime activity. Solutions of CLAFORAN range from very pale yellow
to light amber depending on the concentration and the diluent used. The
pH of the injectable solutions usually ranges from 5.0 to 7.5.
The CAS Registry Number is 64485-93-4.
Indications and Usage
Treatment of infections of lower respiratory tract including pneumonia,
urinary tract, skin and skin structures, bone and joints; treatment of
bacteremia/septicemia, CNS infections, intra-abdominal infections
including peritonitis, gynecological infections including pelvic
inflammatory disease, endometritis and pelvic cellulitis caused by
susceptible strains of specific microorganisms; perioperative
prophylaxis.
Side effects
Cefotaxime may cause side effects. If you are administering cefotaxime
into a muscle, it may be mixed with lidocaine (Xylocaine) to reduce pain
at the injection site. Tell your health care provider if any of these
symptoms are severe or do not go away:
* diarrhea
* stomach pain
* upset stomach
* vomiting
If you experience any of the following symptoms, call your health care
provider immediately:
* unusual bleeding or bruising
* difficulty breathing
* skin rash
* itching
* hives
* sore mouth or throat
Storage/Stability/Compatibility -
Cefotaxime sodium sterile powder for injection should be stored at
temperatures of less than 30°C; protect from light. The commercially
available frozen injection should be stored at temperatures no greater
than -20°C. Depending on storage conditions, the powder or solutions may
darken which may indicate a loss in potency.
Doses -
Horses:
For susceptible infections:
a) Foals: 20 - 30 mg/kg IV q6h (Caprile and Short 1987)
Dosage Forms/Preparations/FDA Approval Status -
Veterinary-Approved Products: None
Human-Approved Products:
Cefotaxime Sodium Powder for Injection; 500 mg, 1 g (as cefotaxime), 2
g, 10 g;
Cefotaxime Sodium for Injection in 5% dextrose bags (50 ml)—frozen; 1 g,
2 g;
|
|








