|
 Description :
Description :
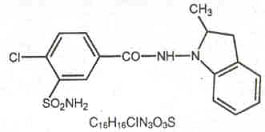
Indapamide is an oral antihypertensive/diuretic. Its molecule
contains both a polar sulfamoyl chlorobenzamide moiety and a
lipid-soluble methylindoline moiety. It differs chemically from
the thiazides in that it does not possess the thiazide ring system
and contains only one sulfonamide group. The chemical name of
indapamide is
4-Chloro-N-(2-methyl-1-indolinyl)-3-sulfamoylbenzamide, and its
molecular weight is 365.84. The compound is a weak acid, pKa =
8.8, and is soluble in aqueous solutions of strong bases. It is a
white to yellow-white crystalline (tetragonal) powder.
Each tablet, for oral administration, contains indapamide 1.25 mg
or 2.5 mg. In addition, each tablet contains the following
inactive ingredients: FD&C Yellow No. 6 (1.25 mg), hypromellose,
lactose monohydrate, magnesium stearate, maize starch,
microcrystalline cellulose, polyethylene glycol, povidone and
titanium dioxide.
 Clinical Pharmacology :
Clinical Pharmacology :
Indapamide is the first of a new class of
antihypertensive/diuretics, the indolines. It has been reported
that the oral administration of 2.5 mg (two 1.25 mg tablets) of
indapamide to male subjects produced peak concentrations of
approximately 115 ng/mL of the drug in blood within two hours. It
has been reported that the oral administration of 5 mg (two 2.5 mg
tablets) of indapamide to healthy male subjects produced peak
concentrations of approximately 260 ng/mL of the drug in the blood
within two hours. A minimum of 70% of a single oral dose is
eliminated by the kidneys and an additional 23% by the
gastrointestinal tract, probably including the biliary route. The
half-life of indapamide in whole blood is approximately 14 hours.
Indapamide is preferentially and reversibly taken up by the
erythrocytes in the peripheral blood. The whole blood/plasma ratio
is approximately 6:1 at the time of peak concentration and
decreases to 3.5:1 at eight hours. From 71 to 79% of the
indapamide in plasma is reversibly bound to plasma proteins.
Indapamide is an extensively metabolized drug with only about 7%
of the total dose administered, recovered in the urine as
unchanged drug during the first 48 hours after administration. The
urinary elimination of 14C-labeled indapamide and metabolites is
biphasic with a terminal half-life of excretion of total
radioactivity of 26 hours.
In a parallel design double-blind, placebo controlled trial in
hypertension, daily doses of indapamide between 1.25 mg and 10 mg
produced dose-related antihypertensive effects. Doses of 5 mg and
10 mg were not distinguishable from each other although each was
differentiated from placebo and 1.25 mg indapamide. At daily doses
of 1.25 mg, 5 mg and 10 mg, a mean decrease of serum potassium of
0.28, 0.61 and 0.76 mEq/L, respectively, was observed and uric
acid increased by about 0.69 mg/100 mL.
In other parallel design, dose-ranging clinical trials in
hypertension and edema, daily doses of indapamide between 0.5 and
5 mg produced dose-related effects. Generally, doses of 2.5 and 5
mg were not distinguishable from each other although each was
differentiated from placebo and from 0.5 or 1 mg indapamide. At
daily doses of 2.5 and 5 mg a mean decrease of serum potassium of
0.5 and 0.6 mEq/Liter, respectively, was observed and uric acid
increased by about 1 mg/100 mL.
At these doses, the effects of indapamide on blood pressure and
edema are approximately equal to those obtained with conventional
doses of other antihypertensive/diuretics.
In hypertensive patients daily doses of 1.25, 2.5 and 5 mg of
indapamide have no appreciable cardiac inotropic or chronotropic
effect. The drug decreases peripheral resistance, with little or
no effect on cardiac output, rate or rhythm. Chronic
administration of indapamide to hypertensive patients has little
or no effect on glomerular filtration rate or renal plasma flow.
Indapamide had an antihypertensive effect in patients with varying
degrees of renal impairment, although in general, diuretic effects
declined as renal function decreased.
In a small number of controlled studies, indapamide taken with
other antihypertensive drugs such as hydralazine, propranolol,
guanethidine, and methyldopa, appeared to have the additive effect
typical of thiazide-type diuretics.
 Indications :
Indications :
Indapamide is indicated for the treatment of hypertension,
alone or in combination with other antihypertensive drugs.
Indapamide is also indicated for the treatment of salt and fluid
retention associated with congestive heart failure.
 Usage in Pregnancy:
Usage in Pregnancy:
The routine use of diuretics in an otherwise healthy woman is
inappropriate and exposes mother and fetus to unnecessary hazard
(see PRECAUTIONS). Diuretics do not prevent development of toxemia
of pregnancy, and there is no satisfactory evidence that they are
useful in the treatment of developed toxemia.
|