|
 Composition
Composition

BRUFADE ®
Deferasirox
Dispersible Tablets,
100,125,250,400 & 500 MG
Each
dispersible tablet contains: Deferasirox. 100 mg, Excipients Q.S.
Each dispersible tablet contains: Deferasirox. 125 mg,
Excipients Q.S.
Each dispersible tablet contains: Deferasirox. 250 mg,
Excipients Q.S.
Each dispersible tablet contains: Deferasirox. 400 mg,
Excipients Q.S.
Each
dispersible tablet contains: Deferasirox. 500 mg, Excipients Q.S.
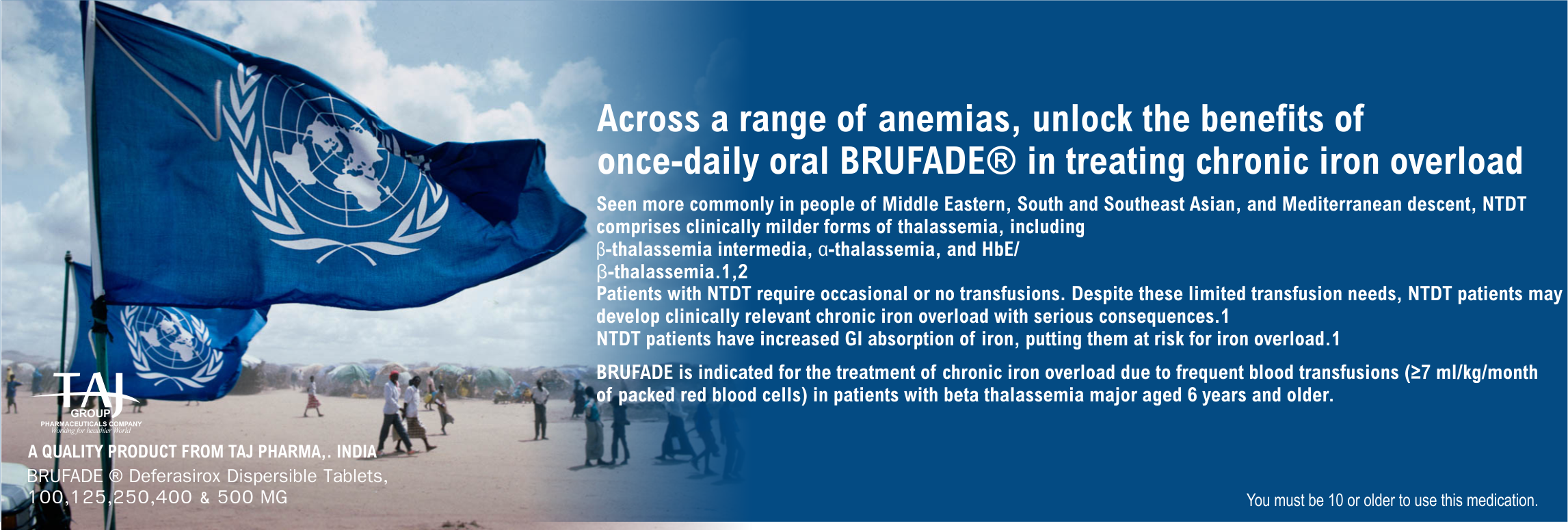
Across a range of anemias, unlock the benefits of once-daily
oral BRUFADE ® in treating chronic iron overload
Brufade (deferasirox) binds
to iron and removes it from the blood stream. Brufade is used to
treat iron overload caused by blood transfusions in adults and
children at least 2 years old.
BRUFADE is a tridentate ligand that binds iron with high
affinity, forming a 2:1 complex that is excreted in bile and
eliminated primarily via the feces.
BRUFADE is indicated for the treatment of chronic iron overload
due to frequent blood transfusions (≥7 ml/kg/month of packed red
blood cells) in patients with beta thalassemia major aged 6
years and older.
BRUFADE is also indicated
for the treatment of chronic iron overload due to blood
transfusions when deferoxamine therapy is contraindicated or
inadequate in the following patient groups:
-
in
patients with beta thalassemia major with iron overload due
to frequent blood transfusions (≥7 ml/kg/month of packed red
blood cells) aged 2 to 5 years
-
in
patients with beta thalassemia major with iron overload due
to infrequent blood transfusions (<7 ml/kg/month of packed
red blood cells) aged 2 years and older
-
in
patients with other anemias aged 2 years and older
 BRUFADE is also indicated for the
treatment of chronic iron overload requiring chelation therapy
when deferoxamine therapy is contraindicated or inadequate in
patients with non−transfusion−dependent thalassemia syndromes
aged 10 years and older.
BRUFADE is also indicated for the
treatment of chronic iron overload requiring chelation therapy
when deferoxamine therapy is contraindicated or inadequate in
patients with non−transfusion−dependent thalassemia syndromes
aged 10 years and older.
Contraindications
 BRUFADE is contraindicated in:
BRUFADE is contraindicated in:
-
patients
with hypersensitivity to the active substance or the
following excipients: lactose monohydrate; crospovidone type
A; cellulose, microcrystalline; povidone; sodium lauryl
sulfate; silica, colloidal anhydrous; magnesium stearate
-
combination with other iron chelator therapies as the safety
of such combinations has not been established
-
patients
with estimated creatinine clearance <60 ml/min
Warning
Data in children with NTDT are very limited. As a consequence,
BRUFADE therapy should be closely monitored to detect side
effects and to follow iron burden in the pediatric population.
In addition, before treating heavily iron-overloaded children
with NTDT with BRUFADE, the physician should be aware that the
consequences of long-term exposure in such patients are
currently not known.
Starting BRUFADE® treatment
in NTDT patients with chronic iron overload
Measure iron overload with
liver iron concentration (LIC)
LIC is the preferred method of iron overload determination and
should be used wherever available. Caution should be taken
during chelation therapy to minimize the risk of overchelation
in all patients.
One course of treatment with BRUFADE is recommended for patients
with NTDT who achieve a satisfactory iron level.
Measure iron overload with
liver iron concentration (LIC)
LIC is the preferred method of iron overload determination and
should be used wherever available. Caution should be taken
during chelation therapy to minimize the risk of overchelation
in all patients.
One course of treatment with
BRUFADE is recommended for patients with NTDT who achieve a
satisfactory iron level.
What is BRUFADE?
BRUFADE (deferasirox) binds to iron
and removes it from the blood stream. BRUFADE is used to
treat iron overload caused by blood transfusions in adults and
children at least 2 years old. BRUFADE is also used to
treat chronic iron overload syndrome caused by a genetic blood
disorder in adults and children who are at least 10 years old.
Important information
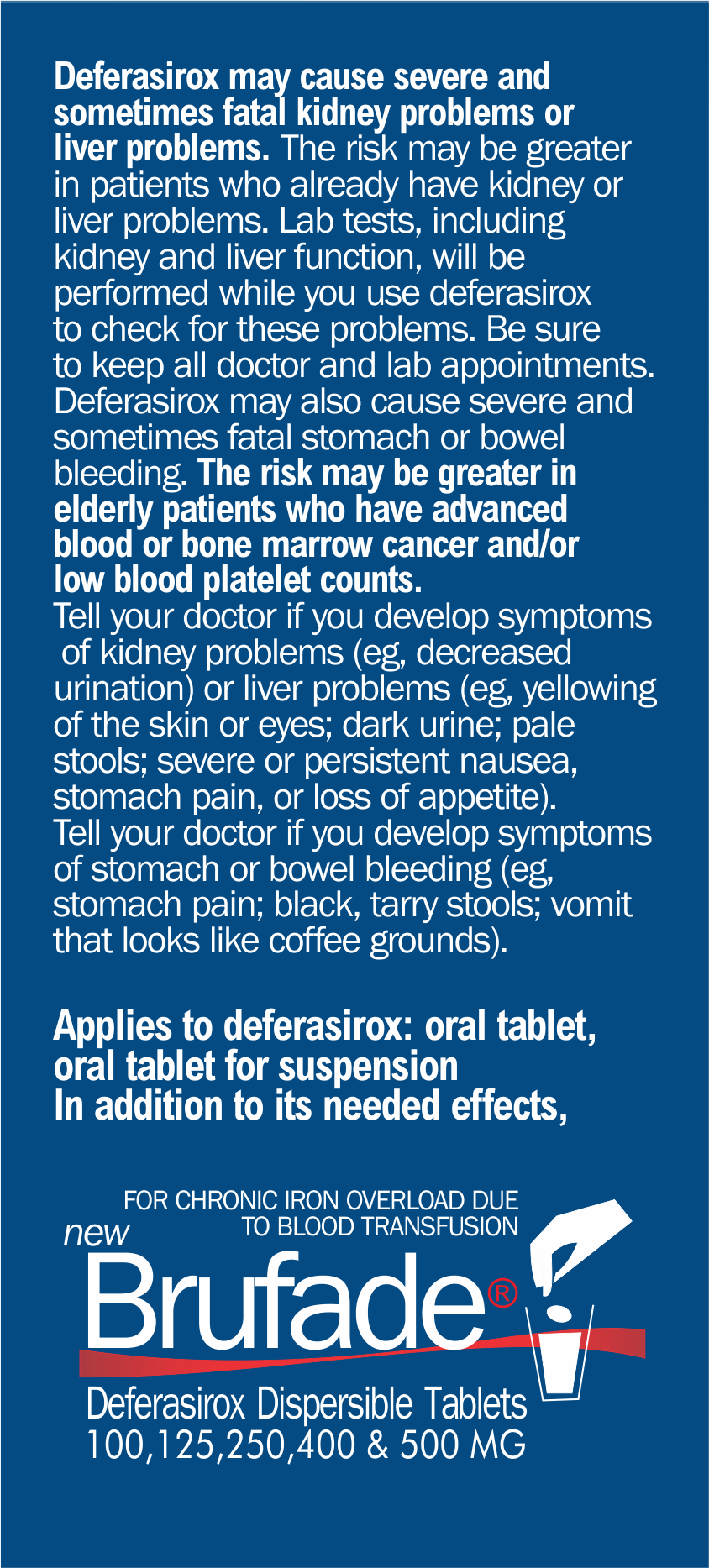
You should not use BRUFADE if you have
severe kidney or liver disease, advanced cancer, a blood cell or
bone marrow disorder, or low levels of platelets in your blood.
Deferasirox can harm your
liver or kidneys. Stop using BRUFADE
and call your doctor at once if you have swelling, rapid weight
gain, shortness of breath, pain in your upper stomach, loss of
appetite, pain in your side or lower back, little or no
urinating, dark urine, clay-colored stools, or jaundice
(yellowing of the skin or eyes). Deferasirox may also cause
stomach or intestinal bleeding. Call your doctor at once if you
have symptoms of stomach bleeding such as bloody or tarry
stools, or coughing up blood or vomit that looks like coffee
grounds. While you are taking BRUFADE, do not take antacids that
contain aluminum, such as Amphojel, Gaviscon, Maalox, Mi-Acid,
Mylanta, Rulox, and others.
Before taking this medicine
You should not use BRUFADE if you are allergic to deferasirox,
or if you have: severe liver or kidney disease;
advanced cancer; a bone marrow disorder; or low levels of
platelets in your blood.
To make sure BRUFADE is safe for you, tell your doctor if you
have:
kidney disease; liver disease; anemia (low red blood cells);
cancer (especially blood cell cancer such as leukemia); a
stomach ulcer; a history of stomach or intestinal bleeding;
vision or hearing problems; or a weak immune system caused by
disease (such as cancer, HIV, or AIDS), or by receiving
steroids, chemotherapy, or radiation. It is not known whether
BRUFADE will harm an unborn baby. Tell your doctor if you are
pregnant or plan to become pregnant.
BRUFADE can make birth
control pills less effective. Ask your doctor about using non
hormonal birth control (condom, diaphragm with spermicide) to
prevent pregnancy.
It is not known whether
deferasirox passes into breast milk or if it could harm a
nursing baby. You should not breast-feed while you are taking
BRUFADE.
How should I take BRUFADE?
Take BRUFADE exactly as prescribed by your doctor. Follow all
directions on your prescription label. Do not take this medicine
in larger or smaller amounts or for longer than recommended.
Your doctor may perform
certain tests to make sure you do not have conditions that would
prevent you from safely using BRUFADE.
Take this medicine at the same time every day.
Take BRUFADE on an empty stomach at least 30 minutes before
eating.
Do not chew or crush the BRUFADE dispersible tablet and do not
swallow it whole. Place the tablet into a glass of apple juice,
orange juice, or water and allow the tablet to disperse in the
liquid. The tablet will not dissolve completely. Drink this
mixture right away. To make sure you get the entire dose, add a
little more liquid to the same glass, swirl gently and drink
right away.
If you take less than 1000
milligrams (1 gram) daily, dissolve the tablet in about one-half
cup of apple juice, orange juice, or water. If you take more
than 1000 milligrams daily, dissolve the tablet in about 1 cup
of apple juice, orange juice, or water.
While using BRUFADE, you may
need frequent blood tests. Your kidney or liver function may
also need to be checked every 6 months, and you may need a liver
biopsy.
Store at room temperature,
away from moisture and heat.

BRUFADE side effects :
Get emergency medical help if you have any of these signs of an
allergic reaction to BRUFADE: hives; difficult breathing;
swelling of your face, lips, tongue, or throat.
Stop using BRUFADE and call your doctor at once if you have:
problems with vision or hearing;
kidney problems - urinating more or less than usual; painful or
difficult urination; swelling in your feet or ankles; weakness,
bone pain; feeling tired or short of breath; liver problems -
nausea, upper stomach pain, itching, tired feeling, loss of
appetite, dark urine, clay-colored stools, jaundice (yellowing
of the skin or eyes); signs of stomach bleeding - bloody or
tarry stools, coughing up blood or vomit that looks like coffee
grounds; low blood cell counts - fever, chills, flu-like
symptoms, swollen gums, mouth sores, skin sores, rapid heart
rate, pale skin, easy bruising, unusual bleeding, feeling
light-headed; or severe skin reaction - fever, sore throat,
swelling in your face or tongue, burning in your eyes, skin pain
followed by a red or purple skin rash that spreads (especially
in the face or upper body) and causes blistering and peeling.
Serious side effects may be more likely in older adults.
Common BRUFADE side effects may include: nausea, vomiting,
stomach pain; diarrhea; or skin rash.
Usual Adult Dose for:
Iron Overload
Thalassemia
Usual Pediatric Dose for:
Iron Overload
Thalassemia
Additional dosage information:
Renal Dose Adjustments
Liver Dose Adjustments
Dose Adjustments
Precautions
Dialysis
Other Comments Usual Adult
Dose for Iron Overload
BRUFADE(R): Initial dose: 20
mg/kg, orally, once a day - calculate dose to the nearest whole
tablet
Monitor serum ferritin monthly: Adjust dose every 3 to 6 months,
by 5 to 10 mg/kg, based on serum ferritin trends
Maximum dose: 40 mg/kg
Comments: -Only consider therapy when evidence of chronic
transfusional overload exists (e.g. transfusion of at least 100
mL/kg packed red blood cells and a serum ferritin consistently
greater than 1000 mcg/L).
-Prior to treatment, obtain
serum ferritin level, baseline serum creatinine in duplicate and
determine creatinine clearance, serum transaminases, bilirubin,
and baseline auditory and ophthalmic examinations.
-Tailor dose to patient
response and therapeutic goals.
Use: Transfusional iron overload
Usual Adult Dose for Thalassemia
BRUFADE(R):
Initial dose: 10 mg/kg, orally, once a day - calculate dose to
the nearest whole tablet
-If baseline liver iron concentration (LIC) is over 15 Fe/g dw,
consider increasing dose to 20 mg/kg/day after 4 weeks
Maximum dose: 20 mg/kg/day
After 6 months therapy:
-If LIC remains above 7 mg
Fe/g dw: increase to 20 mg/kg/day
-If LIC is 3 to 7 mg Fe/g dw: Continue at no more than 10
mg/kg/day

 Presentation Presentation
BRUFADE ® Deferasirox Dispersible Tablets,
100,125,250,400 & 500 MG
Deferasirox Dispersible Tablets
100
ALU- ALU Blister pack of 10 tablets
Deferasirox Dispersible Tablets
125
ALU- ALU Blister pack of 10 tablets
Deferasirox Dispersible Tablets
250
ALU- ALU Blister pack of 10 tablets
Deferasirox Dispersible Tablets
400
ALU- ALU Blister pack of 10 tablets
Deferasirox Dispersible Tablets
500
ALU- ALU Blister pack of 10 tablets
.JPG)
.JPG)
.JPG)
.JPG)
|





.png)