|
|
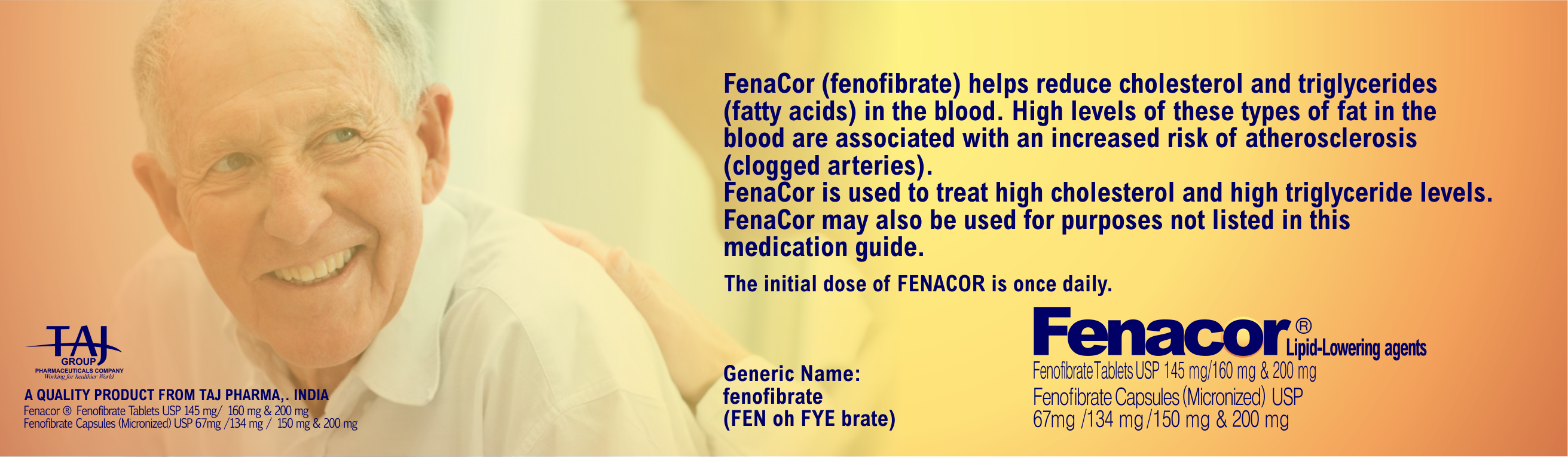
FENACOR Uses and Important Safety Information You
Should Know About FenaCor
Fenacor ®
fenofibrate tablets & Capsules USP 145 mg/ 160 mg & 200 mg
Fenofibrate Capsules (Micronized) USP 67mg /134 mg / 150 mg &
200 mg
Indications
and Usage for Fenacor
Primary Hypercholesterolemia or
Mixed Dyslipidemia
Fenacor
is indicated as adjunctive therapy to diet to reduce elevated
low-density lipoprotein cholesterol (LDL-C), total cholesterol
(Total-C), Triglycerides and apolipoprotein B (Apo B), and to
increase high-density lipoprotein cholesterol (HDL-C) in adult
patients with primary hypercholesterolemia or mixed dyslipidemia.
Severe Hypertriglyceridemia
Fenacor
is also indicated as adjunctive therapy to diet for treatment of
adult patients with severe hypertriglyceridemia. Improving
glycemic control in diabetic patients showing fasting
chylomicronemia will usually obviate the need for pharmacologic
intervention.
Markedly elevated levels of serum
triglycerides (e.g. > 2,000 mg/dL) may increase the risk of
developing pancreatitis. The effect of fenofibrate therapy on
reducing this risk has not been adequately studied.
Important Limitations of Use
Fenofibrate
at a dose equivalent to 145 mg of Fenacor was not shown to
reduce coronary heart disease morbidity and mortality in a
large, randomized controlled trial of patients with type 2
diabetes mellitus [see
Warnings and Precautions.
Fenacor
Dosage and Administration
General Considerations
Patients should be placed on an
appropriate lipid-lowering diet before receiving Fenacor, and
should continue this diet during treatment with Fenacor. Fenacor
tablets can be given without regard to meals.
The initial treatment for
dyslipidemia is dietary therapy specific for the type of
lipoprotein abnormality. Excess body weight and excess alcoholic
intake may be important factors in hypertriglyceridemia and
should be addressed prior to any drug therapy. Physical exercise
can be an important ancillary measure. Diseases contributory to
hyperlipidemia, such as hypothyroidism or diabetes mellitus
should be looked for and adequately treated. Estrogen therapy,
thiazide diuretics and beta-blockers, are sometimes associated
with massive rises in plasma triglycerides, especially in
subjects with familial hypertriglyceridemia. In such cases,
discontinuation of the specific etiologic agent may obviate the
need for specific drug therapy of hypertriglyceridemia.
Uses
- FenaCor® (fenofibrate
tablets & Capsules) is
a prescription medicine used along with diet to lower
triglycerides, total cholesterol, and LDL (bad) cholesterol, and
increase HDL (good) cholesterol.
- FenaCor is also used along with diet to lower
high triglycerides. Excess body weight, drinking alcohol,
diseases such as diabetes and certain thyroid problems, and
various drugs can increase triglyceride levels and should be
evaluated by your doctor before you take FenaCor.
- FenaCor should be used with a diet low in
saturated fat and cholesterol when non-drug measures alone have
not been successful.
Important Safety Information
- FenaCor should not be taken by people with
liver, gallbladder, or severe kidney disease, or those allergic
to any product ingredient.
- FenaCor is associated with increases in liver
enzymes as measured by blood tests. Your healthcare provider may
do blood tests before and during treatment with FenaCor to check
for liver problems.
- Some people who take FenaCor have developed
gallstones. Contact your healthcare provider if you experience
abdominal pain, nausea, or vomiting while taking FenaCor. These
may be signs of inflammation of the gallbladder or pancreas.
- Unexplained muscle pain, tenderness, or
weakness, particularly when occurring with tiredness and fever,
may be a sign of a serious but rare muscle problem and should be
reported to your healthcare provider right away.
- Tell your healthcare provider about all the
medicines you take, including any other cholesterol medications,
to determine if the combination of these medicines is right for
you.
- FenaCor has not been shown to prevent heart
disease or heart attack.
- FenaCor may cause allergic-type reactions and
possible changes in blood chemistry.
- The most common side effects with FenaCor
include stomach pain, back pain, headache, increases in liver
enzymes measured by blood tests, and respiratory symptoms.
This is the most important information to know
about FenaCor. For more information, talk with your healthcare provider.

Indications and Important Safety Information
About FenaCor
FenaCor Type IIa/IIb
Indications
- FenaCor is indicated as
adjunctive therapy to diet in adult patients with primary
hypercholesterolemia or mixed dyslipidemia (Fredrickson types
IIa and IIb) to: increase high-density lipoprotein cholesterol (HDL-C),
reduce triglycerides (TG), reduce low-density lipoprotein
cholesterol (LDL-C), reduce total cholesterol (Total-C), reduce
apolipoprotein B (Apo B).
- Lipid-altering agents should be used in
addition to a diet restricted in saturated fat and cholesterol
when response to diet and nonpharmacological interventions alone
has been inadequate.
FenaCor Type IV/V
Indications
- FenaCor is indicated as
adjunctive therapy to diet for treatment of adult patients with
hypertriglyceridemia (Fredrickson types IV and V hyperlipidemia).
Improving glycemic control in diabetic patients with fasting
chylomicronemia usually obviates the need for drug therapy.
- The effect of FenaCor on pancreatitis risk
reduction in patients with markedly elevated serum TG has not
been adequately studied.
- Drug therapy is not indicated for patients
with type I hyperlipoproteinemia.
- The use of FenaCor should be considered only
when reasonable attempts have been made to obtain satisfactory
results with nondrug methods such as diet, exercise, and
reduction of alcohol intake. If the decision is made to use
FenaCor, the patient should be instructed that this does not
reduce the importance of adhering to diet. Diseases or drugs
which contribute to hyperlipidemia should be evaluated and
appropriate interventions begun.
Important Safety Information
- FenaCor is
contraindicated in patients with: hypersensitivity to
fenofibrate; hepatic or severe renal dysfunction including
primary biliary cirrhosis; unexplained persistent liver function
abnormality; and preexisting gallbladder disease.
- Fenofibrate has been
associated with increases in serum transaminases. Regular liver
function monitoring should be performed, and therapy
discontinued if enzyme levels persist >3 times the normal limit.
- Fenofibrate may lead to
cholelithiasis. If cholelithiasis is confirmed, FenaCor should
be discontinued.
- FenaCor may increase
the effects of coumarin-type anticoagulants. Dosage adjustment
based on frequent prothrombin time/INR determinations is
advisable.
- The combined use of FenaCor and HMG-CoA
reductase inhibitors should be avoided unless the benefit of
further alterations in lipid levels is likely to outweigh the
increased risk. This combination has been associated with
rhabdomyolysis, markedly elevated creatine kinase levels and
myoglobinuria, leading to acute renal failure.
- FenaCor may
occasionally be associated with myositis, myopathy, or
rhabdomyolysis. Muscle pain, tenderness, or weakness should have
prompt medical evaluation. Discontinue FenaCor if markedly
elevated CPK levels occur or myopathy/myositis is suspected or
diagnosed.
- The effect of FenaCor on coronary heart
disease morbidity and mortality and noncardiovascular mortality
has not been established.
- Other precautions include increases in serum
creatinine that tend to be reversible, pancreatitis,
hypersensitivity reactions, venothromboembolic events, and
hematologic changes.
- Adverse events most frequently observed in
clinical trials: abnormal liver function tests; respiratory
disorder; abdominal pain; back pain; and headache.
Presentation
Fenofibrate Tablets USP 145 mg
10 TABLETS BLISTER
Fenofibrate Tablets USP 160 mg
10 TABLETS BLISTER
Fenofibrate Tablets USP 200 mg
10 TABLETS BLISTER
Fenofibrate Capsules (Micronized) USP 67mg
Fenofibrate Capsules (Micronized) USP 134 mg
10 TABLETS BLISTER
Fenofibrate Capsules (Micronized) USP 150 mg
10 TABLETS BLISTER
Fenofibrate Capsules (Micronized) USP 200 mg
10 TABLETS BLISTER

|
|