|
|
Composition
ALTRAM ®
capsule: Each capsule contains Tramadol Hydrochloride BP 50 mg.
ALTRAM ®100 Injection: Each 2 ml injection contains Tramadol
Hydrochloride BP 100 mg.
ALTRAM ® 50 Injection: Each 1 ml injection contains Tramadol
Hydrochloride BP 50 mg.
ALTRAM
® 100 suppository: Each suppository contains Tramadol
Hydrochloride BP 100 mg.
Pharmacology
ALTRAM ®
(Tramadol) is a centrally acting synthetic analgesic compound.
It inhibits the re
uptake of neurotransmitters- serotonin and noradrenaline. Thus
it modifies the
transmission of pain impulses by activating both descending
serotonergic pathways and
noradrenergic pathways involved in analgesia. The analgesic
effects of Tramadol are
mediated via stimulation of mu-opioid receptors and indirect
modulation of central
monoaminergic inhibitory pathways.
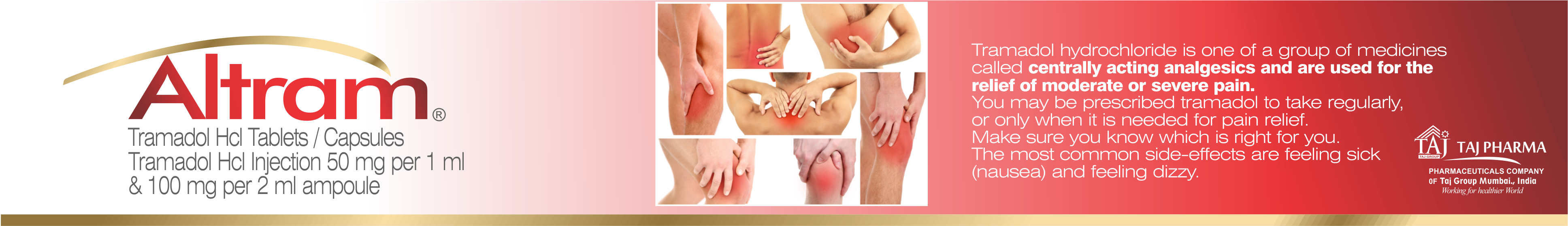
Indication
ALTRAM ®
(Tramadol) is used for the treatment of moderate to severe
painful conditions.
These include:
Ø Post-operative pain
Ø Colic and spastic pain
Ø Cancer pain
Ø Joint pain
Ø Neck and back pain
Ø Pain associated with osteoporosis
Ø Chest pain (including pain associated with myocardial
infarction and angina) etc.
Dosage and Administration
ALTRAM ®
capsule: Usual doses are 50 to 100 mg every four to six hours.
For acute pain an initial dose of
100 mg is required. For chronic painful conditions as initial
dose of 50 mg is
recommended. Subsequent doses should be 50 to 100 mg
administered 4-6 hourly. The
dose level and frequency of dosing will depend on the severity
of the pain. The total
daily dosage by mouth should not exceed 400 mg.
ALTRAM ® injection:
A dose of 50-100 mg may be given every 4 to 6 hours by
intramuscular or by
intravenous infusion. For the treatment of post-operative pain,
the initial dose is 100 mg
followed by 50 mg every 10 to 20 minutes if necessary to a
maximum of 250 mg in the
first hour. Thereafter, doses are 50 to 100 mg every 4 to 6
hours up to a total daily dose
of 600 mg.
ALTRAM ®
suppository:
ALTRAM ® suppository should be administered rectally. For adults
usual dose is one
suppository 6 hourly. In general, 400 mg Tramadol Hydrochloride
(= 4 ALTRAM ® suppository) per day sufficient. However, for the
treatment of Cancer pain and severe
pain after operations much higher daily doses can be used.
Side-Effects
Commonly occurring side-effects are dizziness/vertigo, nausea,
constipation, headache,
somnolence, vomiting, pruritus, CNS stimulation, asthenia,
sweating, dyspepsia, dry
mouth, diarrhoea.
Less commonly occurring side-effects include malaise, allergic
reaction, weight loss,
vasodilatation, palpitations, abdominal pain, anorexia,
flatulence, GI bleeding, hepatitis,
stomatitis etc.
Contraindication
Tramadol is contraindicated in persons having hypersensitivity
to this drug. It is also
contraindicated in acute intoxication with alcohol, hypnotics,
centrally acting analgesics,
opioids or psychotropic drugs.
Drug Interaction
In general, physician need not be concerned about drugs
interacting with Tramadol. The
monoamine oxidase (MAO) inhibitors represent the only drug class
not recommended
for combination with Tramadol.
Concomitant administration of carbamazepine with Tramadol causes
a significant
increase in Tramadol metabolism and it requires to increase the
dose of Tramadol.
Precaution
Respiratory depression: When large doses of tramadol are
administered with
anaesthetic with anaesthetic medications or alcohol, respiratory
depression may result.
Therefore, tramadol should be administered cautiously in
patients at risk for respiratory depression. Opioid dependence:
Tramadol is not recommended for patients who are dependent on
opioids. Concomitant CNS depressants: Tramadol should be used
with caution and in reduced dosages when administering to
patients receiving CNS depressants such as alcohol, opioids,
anesthetic agents, phenothiazines, tranquilizers or sedative
hypnotics.
Concomitant MAO inhibitors : Tramadol should be used with great
caution in patients taking MAO inhibitors, since tramadol
inhibits the uptake of norepinephrine and
serotonin. Tramadol should be used with caution in patients with
increased intracranial pressure or head injury and patients with
acute abdominal conditions.
Use in Pregnancy and Lactation
Safe use of tramadol in pregnancy has not been established.
Tramadol has been shown to cross the placenta. There are no
adequate and well controlled studies in pregnant women.
Therefore, tramadol should be used during pregnancy only if the
potential benefit justifies the risk to the foetus.
Use in Children
In children from the age of one year ALTRAM (Tramadol) can be
given in a dose of 1-2 mg/kg body weight. However, on account of
the dosage strength of ALTRAM suppository (100 mg Tramadol
Hydrochloride) should not be administered in children and
adolescents below the age of
14 years.
How Supplied
ALTRAM®capsule: Box containing 3x10’s capsules in blister pack.
ALTRAM® 100 injection: Each box containing 1x5’s ampoules in
blister pack.
ALTRAM ®50 injection: Each box containing 1x5’s ampoules in
blister pack.
ALTRAM ® 100 suppository : Box containing 1x5’s suppositories in
blister pack.
® Registered Trade Mark TAJ PHARMA
|
|