|
 DRUG DESCRIPTION :
DRUG DESCRIPTION :
NAPROXEN SODIUM Tablets contain naproxen sodium, a
member of the arylacetic acid group of nonsteroidal
anti-inflammatory drugs (NSAIDs). NAPROXEN SODIUM Tablets use the
proprietary IPDAS (Intestinal Protective Drug Absorption System)
technology. It is a rapidly disintegrating tablet system combining
an immediate release component and a sustained release component
of microparticles that are widely dispersed, allowing absorption
of the active ingredient throughout the gastrointestinal (GI)
tract, maintaining blood levels over 24 hours. The chemical name
for naproxen sodium is 2-naphthaleneacetic acid,
6-methoxy-a-methyl-sodium salt, (S)- with the
 following structural formula:
following structural formula:
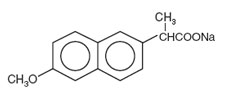
Naproxen sodium
Molecular Formula: C14H13NaO3 Molecular Weight: 252.24
Naproxen sodium is an odorless crystalline powder, white to creamy
in color. It is soluble in methanol and water. NAPROXEN SODIUM
Tablets contain 412.5 mg or 550 mg of naproxen sodium, equivalent
to 375 mg and 500 mg of naproxen and 37.5 mg and 50 mg sodium
respectively. Each NAPROXEN SODIUM Tablet also contains the
following inactive ingredients: ammoniomethacrylate copolymer Type
A, ammo-niomethacrylate copolymer Type B, citric acid,
crospovidone, magnesium stearate, methacrylic acid copolymer Type
A, microcrystalline cellulose, povidone, and talc. The tablet
coating contains hydrox-ypropyl methylcellulose, polyethylene
glycol, and titanium dioxide.
 INDICATIONS :
INDICATIONS :
Carefully consider the potential benefits and risks of NAPROXEN
SODIUM Tablets and other treatment options before deciding to use
NAPROXEN SODIUM Tablets. Use the lowest effective dose for the
shortest duration consistent with individual patient treatment
goals (see WARNINGS).
NAPROXEN SODIUM Tablets are indicated for the treatment of
rheumatoid arthritis, osteoarthritis, ankylosing spondylitis,
tendinitis, bursitis and acute gout. It is also indicated in the
relief of mild to moderate pain and the treatment of primary
dysmenorrhea.
 DOSAGE AND ADMINISTRATION :
DOSAGE AND ADMINISTRATION :
Carefully consider the potential benefits and risks of NAPROXEN
SODIUM and other treatment options before deciding to use NAPROXEN
SODIUM . Use the lowest effective dose for the shortest duration
consistent with individual patient treatment goals (see WARNINGS).
After observing the response to initial therapy with NAPROXEN
SODIUM , the dose and frequency should be adjusted to suit an
individual patient's needs
 SIDE EFFECTS :
SIDE EFFECTS :
As with all drugs in this class, the frequency and severity of
adverse events depends on several factors: the dose of the drug
and duration of treatment; the age, the sex, physical condition of
the patient; any concurrent medical diagnoses or individual risk
factors. The following adverse reactions are divided into three
parts based on frequency and whether or not the possibility exists
of a causal relationship between drug usage and these adverse
events. In those reactions listed as "Probable Causal
Relationship" there is at least one case for each adverse reaction
where there is evidence to suggest that there is a causal
relationship between drug usage and the reported event.
The adverse reactions reported were based on the results from two
double-blind controlled clinical trials of three months duration
with an additional nine month open-label extension. A total of 542
patients received NAPROXEN SODIUM Tablets either in the
double-blind period or in the nine month open-label extension. Of
these 542 patients, 232 received NAPROXEN SODIUM Tablets, 167 were
initially treated with Naproxen and 143 were initially treated
with placebo. Adverse reactions reported by patients who received
NAPROXEN SODIUM Tablets are shown by body system. Those adverse
reactions observed with naproxen but not reported in controlled
trials with NAPROXEN SODIUM Tablets are italicized.
The most frequent adverse events from the double-blind and
open-label clinical trials were headache (15%), followed by
dyspepsia (14%), and flu syndrome (10%). The incidence of other
adverse events occurring in 3% - 9% of the patients are marked
with an asterisk.
 OVERDOSE :
OVERDOSE :
Significant naproxen overdosage may be characterized by
drowsiness, heartburn, indigestion, nausea or vomiting. Because
naproxen sodium may be rapidly absorbed, high and early blood
levels should be anticipated. A few patients have experienced
seizures, but it is not clear whether or not these were
drug-related. It is not known what dose of the drug would be life
threatening.
The oral LD50 of the drug is 500 mg/kg in rats, 1200 mg/kg in
mice, 4000 mg/kg in hamsters and greater than 1000 mg/kg in dogs.
In animals 0.5 g/kg of activated charcoal was effective in
reducing plasma levels of naproxen. Patients should be managed by
symptomatic and supportive care following an NSAID overdose. There
are no specific antidotes. Hemodialysis does not decrease the
plasma concentration of naproxen because of the high degree of its
protein binding. Emesis and/or activated charcoal (60 to 100 g in
adults, 1 to 2 g/kg in children) and/or osmotic carthartic may be
indicated in patients seen within 4 hours of ingestion with
symptoms or following a large overdose. Forced diuresis,
alkalinization of urine or hemoperfusion may not be useful due to
high protein binding.
|







